EMD reports promising PTSD recovery data 6 months after treatment
Our Investment Emyria (ASX:EMD) develops and delivers new treatments for mental health and select neurological conditions.
EMD provides MDMA assisted therapy for post-traumatic stress disorder (PTSD) in its clinics.
The sector is gaining traction with large insurers, as evidenced by Medibank Private committing to invest $50M into mental health.
That funding will support a raft of initiatives including an innovative trial involving psychedelics.
Medibank has allocated $10M to a psychotherapy program for eligible customers with acute mental health conditions such as PTSD, collaborating with the Australian National University (more on ANU below) to look at clinical outcomes as well as broader economic impacts.
Up until now, the main concerns around EMD’s type of therapy were around the durability of the benefits - do the measured benefits recorded at the time of treatment last even months after treatment?
Today EMD has released results confirming they do.
The first 8 patients treated have now reached a period of 6 months after treatment, and the results show the benefits of EMD’s MDMA assisted therapy are durable.
We think that this “durability of impact” would be very important to funders (like health insurers) when evaluating the value proposition of EMD’s treatments.
6 month results in - First 8 patients show strong outcomes:
PTSD symptoms down
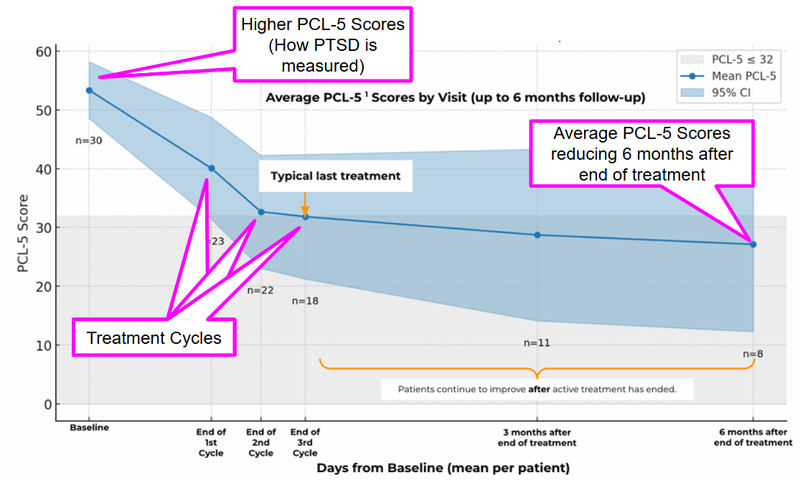
63% of patients no longer met the PTSD criteria, with symptoms dropping 55.5% on average.
(Source)
Average PTSD scores (PCL-5) dropped from baseline to 6 months post-treatment. PCL-5 is a 20-question self-assessment - the higher the score, the worse the symptoms are.
Quality of life up
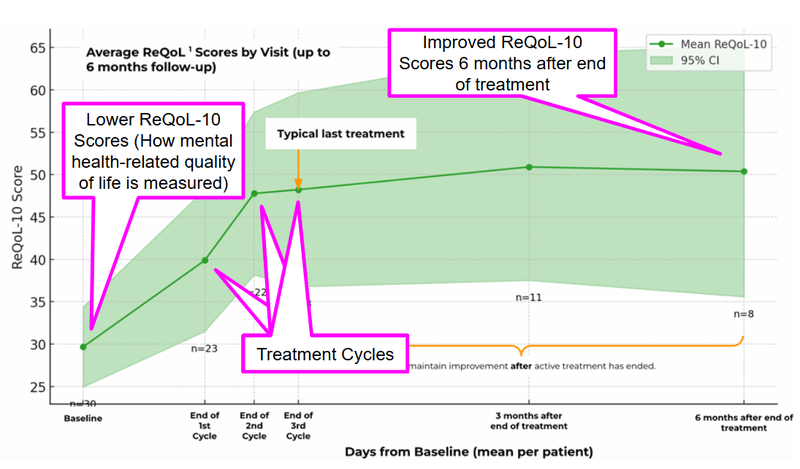
63% reached general population levels, with Recovering Quality of Life (ReQoL) scores improving by 121.5%.
(Source)
Average ReQoL-10 scores from baseline to 6 months post-treatment show strong gains in mental health-related quality of life following MDMA-assisted therapy.
The higher the score, the better the well-being.
The general population typically records a score of around 50% so this shows EMD’s treatments are getting patients back to those levels which is a big achievement.
Lasting Benefits
Improvements held steady six months after treatment in a tough-to-treat group.
These outcomes were achieved in a real-world clinical setting, without a placebo or control group, among patients who had previously failed to respond to conventional treatment.
ANU partnership to advance clinical validation and real world impact
EMD has also signed an MoU with the Australian National University (ANU) to help advance clinical validation, clinician training, and access to future government and insurer-backed mental health care initiatives.
The partnership will:
- Co-develop evidence-based clinical protocols for advanced mental health therapies
- Support clinician training through professional development frameworks
- Leverage ANU’s role as an evaluator in Medibank’s $50M psychotherapy initiative
- Facilitate collaborative research with Professor Paul Fitzgerald, a globally recognised leader in psychiatry and head of ANU’s School of Medicine and Psychology.
While non-binding, the MoU reflects a shared 3-year commitment (May 2025 - May 2028) to accelerate patient access to novel treatments by aligning clinical innovation with academic rigour.
What’s Next for EMD?
Advanced discussions with health funders - could we see a deal in the coming weeks?
In EMD’s most recent quarterly, the company revealed it was in advanced talks with private health insurers, workers’ compensation schemes, and government agencies for funding agreements.
The relatively high cost of EMD’s treatment can be a barrier for suffering patients.
Getting a large organisation like an insurer to cover the costs of the treatment would unlock access to a much larger patient pool for EMD, with treatments covered under funding by insurers as opposed to patients themselves.
The $50M commitment by Medibank Private confirms that large insurers are taking mental health seriously, and investing in models of innovative care.
In EMD’s most recent quarterly, the company flagged that the first funded pilot programs are set to launch before H2 2025, supporting wider access and generating recurring revenue.
That means we could get news on some kind of funded pilot programme in the coming weeks.
We think this could be a material share price catalyst for EMD.
A large deal would drive meaningful revenue growth as EMD ramps up treatment delivery.
Today’s results are helping to validate EMD’s trauma-informed care model and are playing a key role in shaping partnerships with health funders.
New Empax Centre opened, east coast expansion underway
EMD has just opened its second Empax Centre, co-located at Perth Clinic, boosting treatment capacity by 50% and meeting key requirements from major health funders.
With patients now being treated and additional east coast centres in the pipeline, every patient contributes to a growing dataset that supports clinical refinement, funder negotiations, and new IP development - driving value across EMD’s clinical and treatment programs.
Scale clinical health services & engage payers
🔲 Open a new clinic
🔲 Licence a care model
🔲 Secure a major payer
The strong 6-month PTSD outcomes and the MOU with ANU validate EMD’s care model, supporting plans to open new clinics and license their approach.
These real-world results and academic backing also strengthen EMD’s position to secure major payer agreements and expand patient access.
EMD presenting at Psychedelic Science 2025
EMD’s Executive Director and Chief Scientific Officer, Dr. Michael Winlo, will be presenting their promising PTSD treatment findings at Psychedelic Science 2025 in Denver - organised by Multidisciplinary Association for Psychedelic Studies (MAPS).
MAPS are the leading nonprofit advancing psychedelic research - making the conference an important event for the year.
We think EMD is delivering world first treatment models so we are looking forward to seeing what kind of the reception the company might get in Denver.






